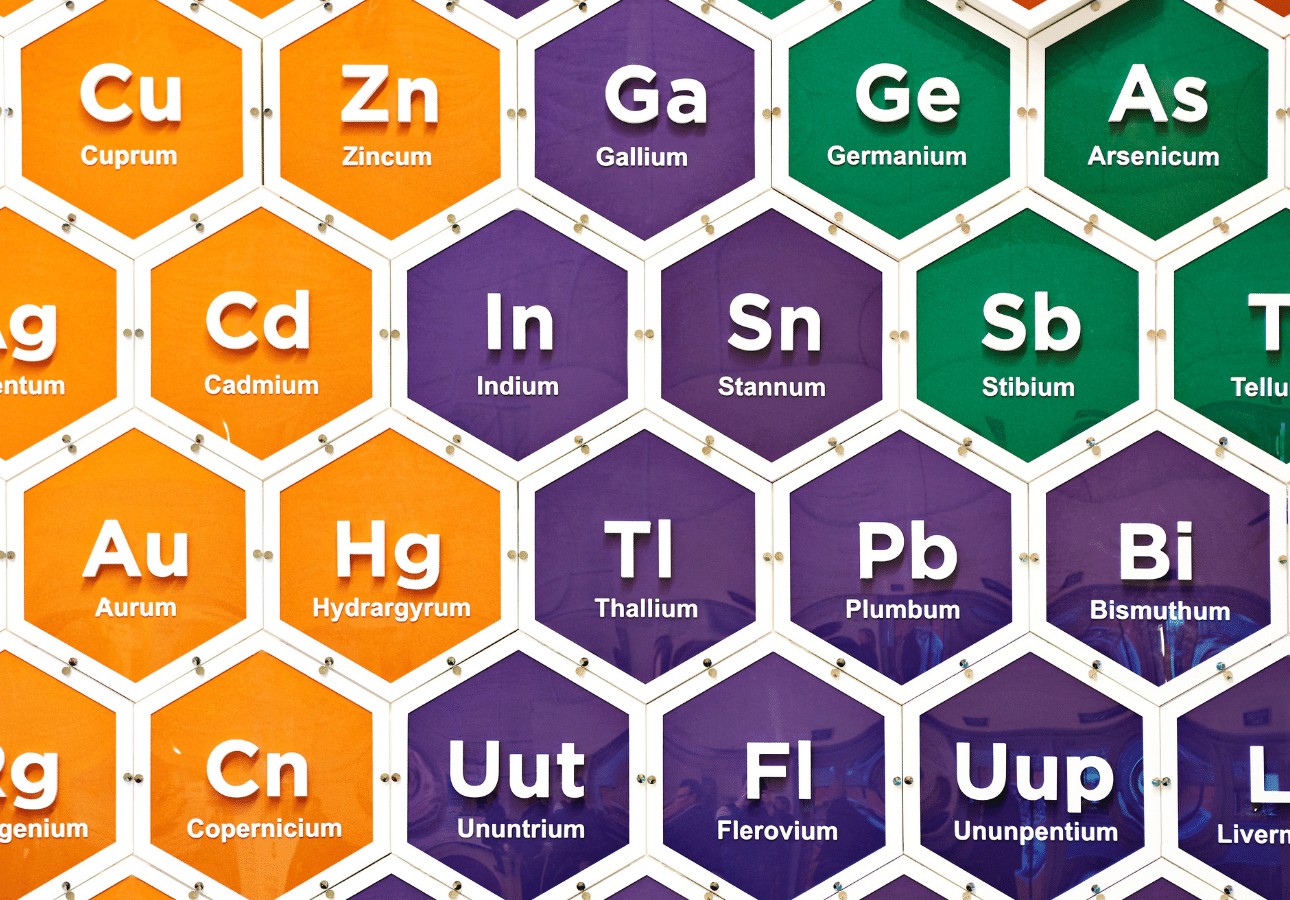
Absolutely everything in the world (including your body) is made up of different combinations of chemicals. The periodic table shows all of the 118 chemical elements that are the building blocks of life as we know it.
The periodic table is actually one of the most important tools that modern scientists have! The history of science is filled with stories of researchers studying chemicals. Even thousands of years ago there were people who understood how important they were. But, it’s not until the last few hundred years that scientists have really come to understand how chemicals work and how they shape the universe that’s all around us.
What is the Periodic Table?
Although it looks a bit complicated, the periodic table is just a system that’s used to arrange the 118 known chemical elements.
The chemical elements on the periodic table are the basic building blocks of all matter in the universe. If it exists, it’s made of chemical elements, and the periodic table is a good way of sorting those elements into their different types. You see, chemical elements aren’t just listed in any random order. The periodic table is laid out according to the Atomic Number of the elements.

To understand atomic numbers we have to take a step back and think about atoms. Everything in the world is made up of chemicals, and every chemical is made up of tiny little particles called atoms. If you could put a single atom under a microscope and zoom in far enough, you’d see that it’s actually made up of even smaller particles called protons, neutrons and electrons. Protons and neutrons join together to become the atom’s nucleus. The nucleus then has one or more electrons bound to it. Depending on the chemical element, each atom will be made up of different numbers of protons, neutrons and electrons.
The atomic number of a chemical is simply the number of protons in the atom. So, Hydrogen (which is the first element on the top left hand side of the periodic table) is assigned the number 1 because it only has one proton in each atom. The periodic table is read from left to right, top to bottom. The atomic number of each element increases as you go along the rows and down the columns. But, rather than just arranging the chemicals right next to each other, they’re also grouped with other chemicals that have similar properties to make the table easier to read.
When Was the Periodic Table Invented?
Chemistry is one of the oldest types of science in the world. People have been studying it for thousands of years in fact! For a long time though, we didn’t really have the right technology to understand chemicals and how they interact with each other. Even though humans lacked technology for a long time, some of history’s greatest discoveries came from people experimenting with chemical elements.

Compared to those earliest researchers, the periodic table is a very new invention. It took almost 100 years of study for chemists from across the world to finally organise the elements, beginning in 1789 with French chemist Antoine Lavoisier. Other chemists built on Antoine’s works, but it wasn’t until 1860 that the atomic mass of every element was published for scientists all over the world to use. In 1869, it was Russian chemist Dmitri Mendeleev who used the new list of atomic masses to create the first version of the modern periodic table.
Since then, scientists have been improving on Dmitri’s periodic table, adding new information and elements as they’re discovered by researchers!
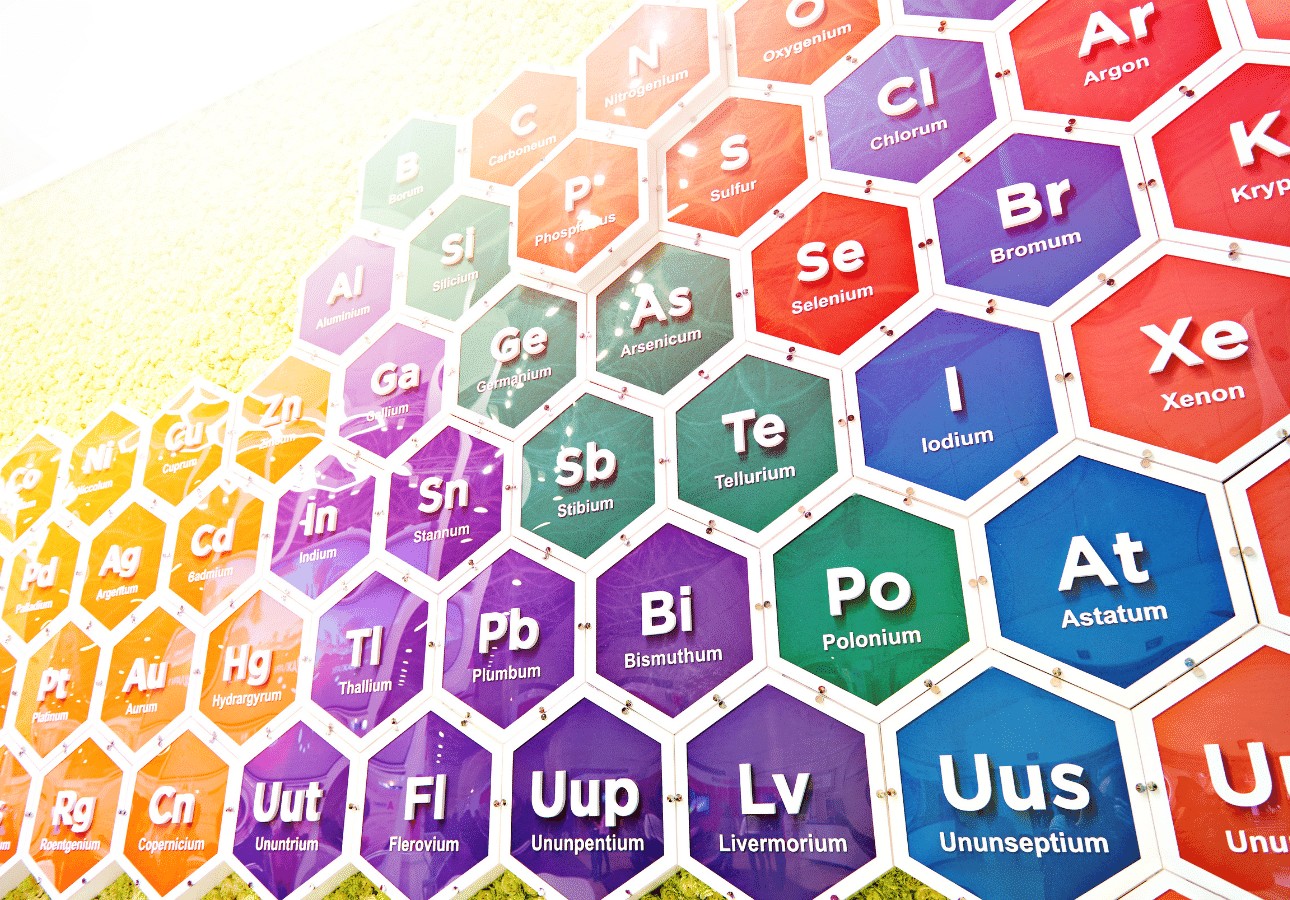
Why is it Called the Periodic Table?
You might be thinking that the periodic table has a bit of a weird name since it shows chemical elements and not periods of time. But the name is actually pretty simple. It’s called the periodic table because of the way the elements are arranged.

The table is arranged into columns and rows. The vertical columns are called ‘groups’ and the horizontal rows are called ‘periods’. Elements that show up in the same groups (columns) have similar properties. For example, the column to the far right is made up of noble gases – a group of gases that are very stable (which means they don’t react much to other elements). Elements that show up in the same periods (rows) have the same number of electrons. Going along the periods from left to right, each element has one more proton and is less metallic than the element that comes before it.
Interested in Learning More About Chemistry? Book a Show With Street Science!
Chemistry powers the whole world. It’s one of the most exciting scientific fields because we’re always learning something new! If you’d like to get in on the fun then Street Science has the perfect courses! Our Perfect Polymers classes for years 2, 5 and 6 dive deeper into chemistry and how it helps make our world go around. All of our teachers are highly experienced, and they know how to deliver classes that are as fun as they are educational. Book a class with us online, or have a look at our Classroom Kits if you’d like to bring a little bit of Street Science to your next class!